BC Platforms brings agentic AI to real-world data
BC Platforms in the press: Signify Research reviews BC Catalyst, our AI-powered platform for life sciences and precision medicine.

Designed for secure, compliant, and scalable research, BC Mosaic is our AI-powered trusted research environment (TRE) for clinical, genomic, and multi-omics studies.
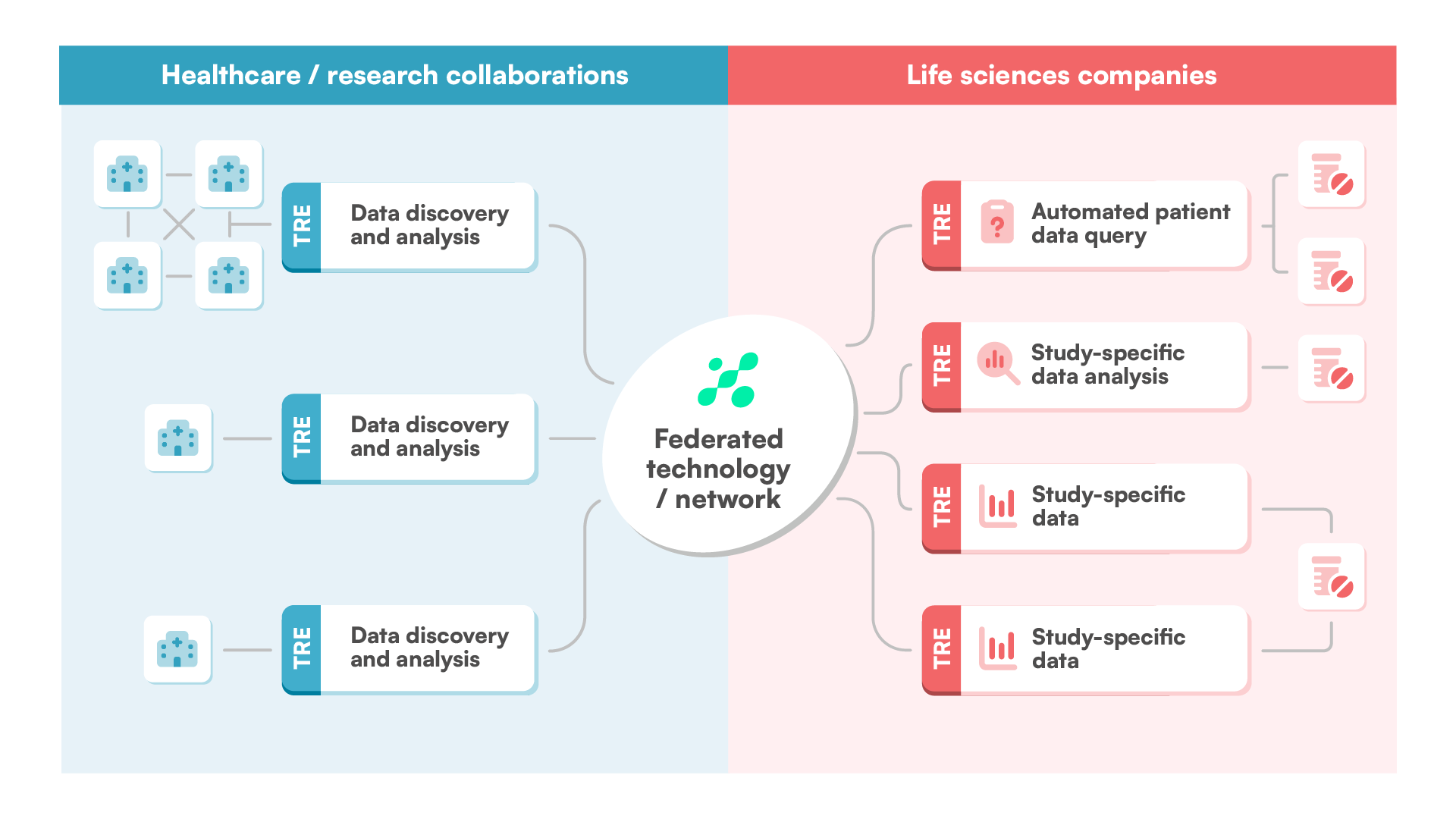
BC Mosaic provides secure, compliant access to diverse datasets in a trusted research environment, enabling efficient and collaborative analysis across countries and organizations through federated architecture and AI-powered tools.
Scale up your data management, analytics and insights with BC Mosaic, which centralizes multi-modal data management, empowers intuitive cohort discovery and planning, and accelerates advanced analytics with robust governance and AI-powered tools. Built for GDPR+ compliance and ready for European Health Data Space (EHDS) regulations.
Centralize data management and access to harmonized, research-ready data in one federated platform — allowing you to focus on generating insights without worrying about data integration or security.
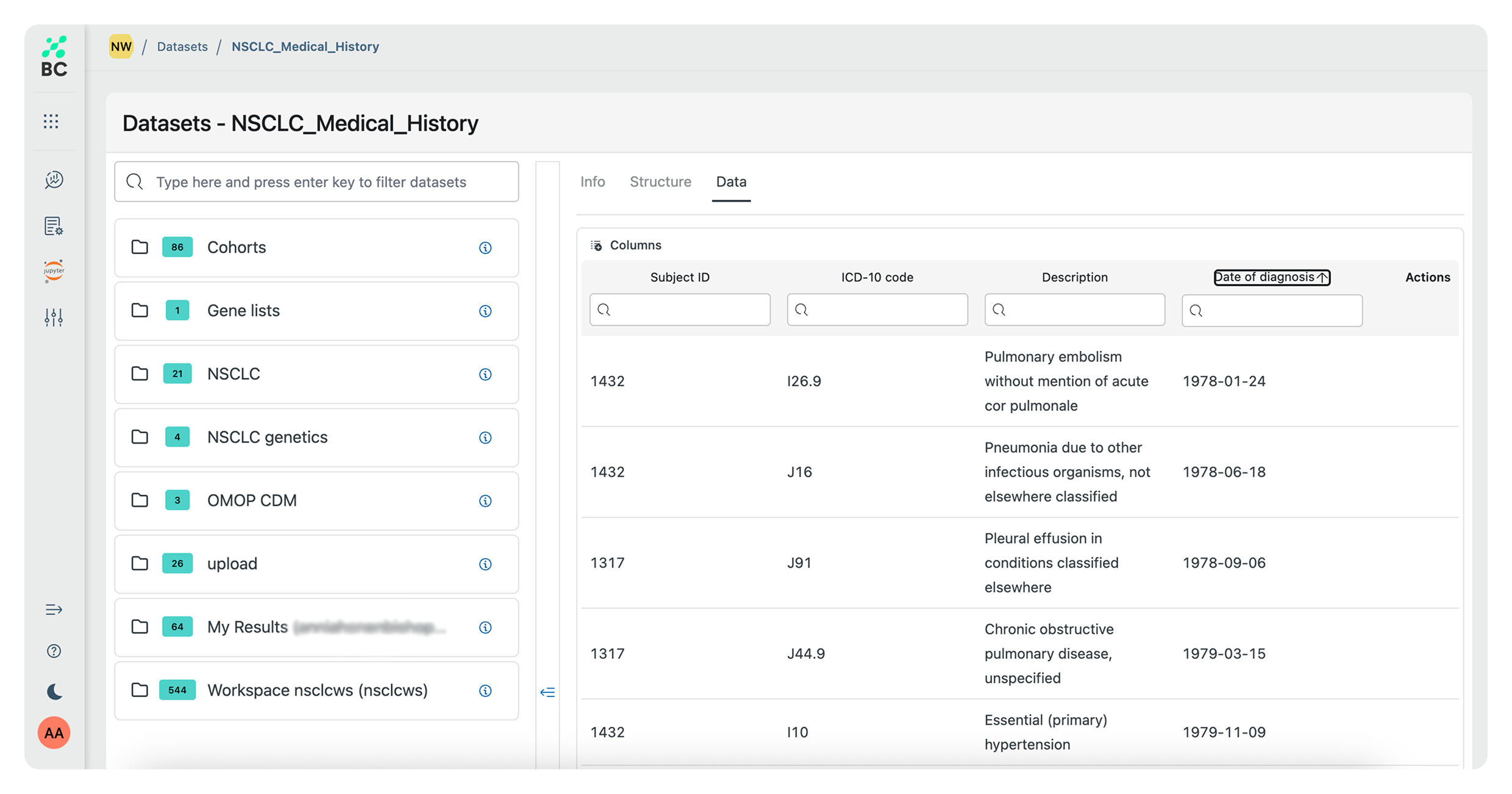
Use easy and intuitive no-code tools for complex queries on clinical and genetic data for a variety of research needs. Identify cohorts at a global level, see which sites have the data, and submit data requests.
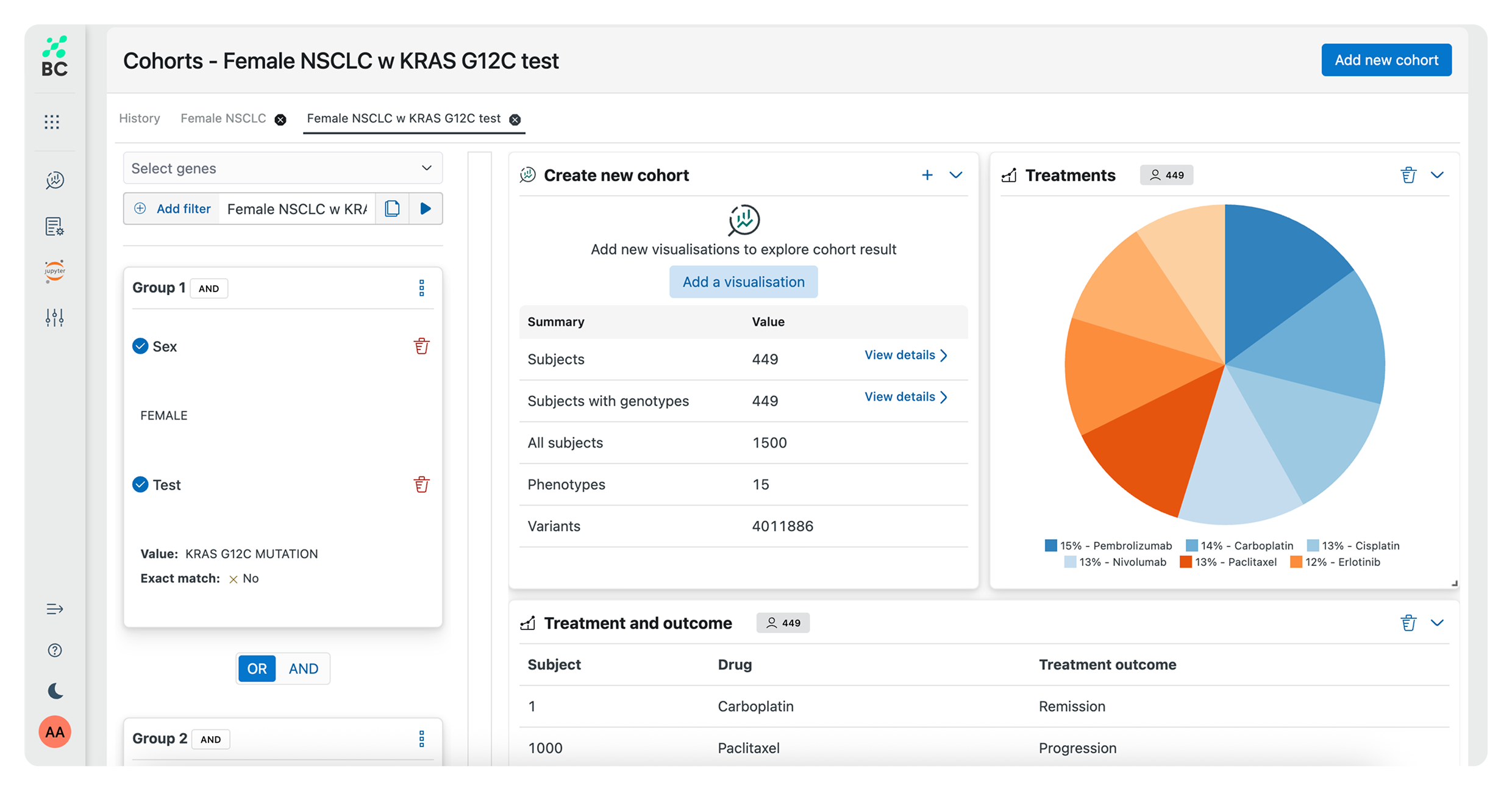
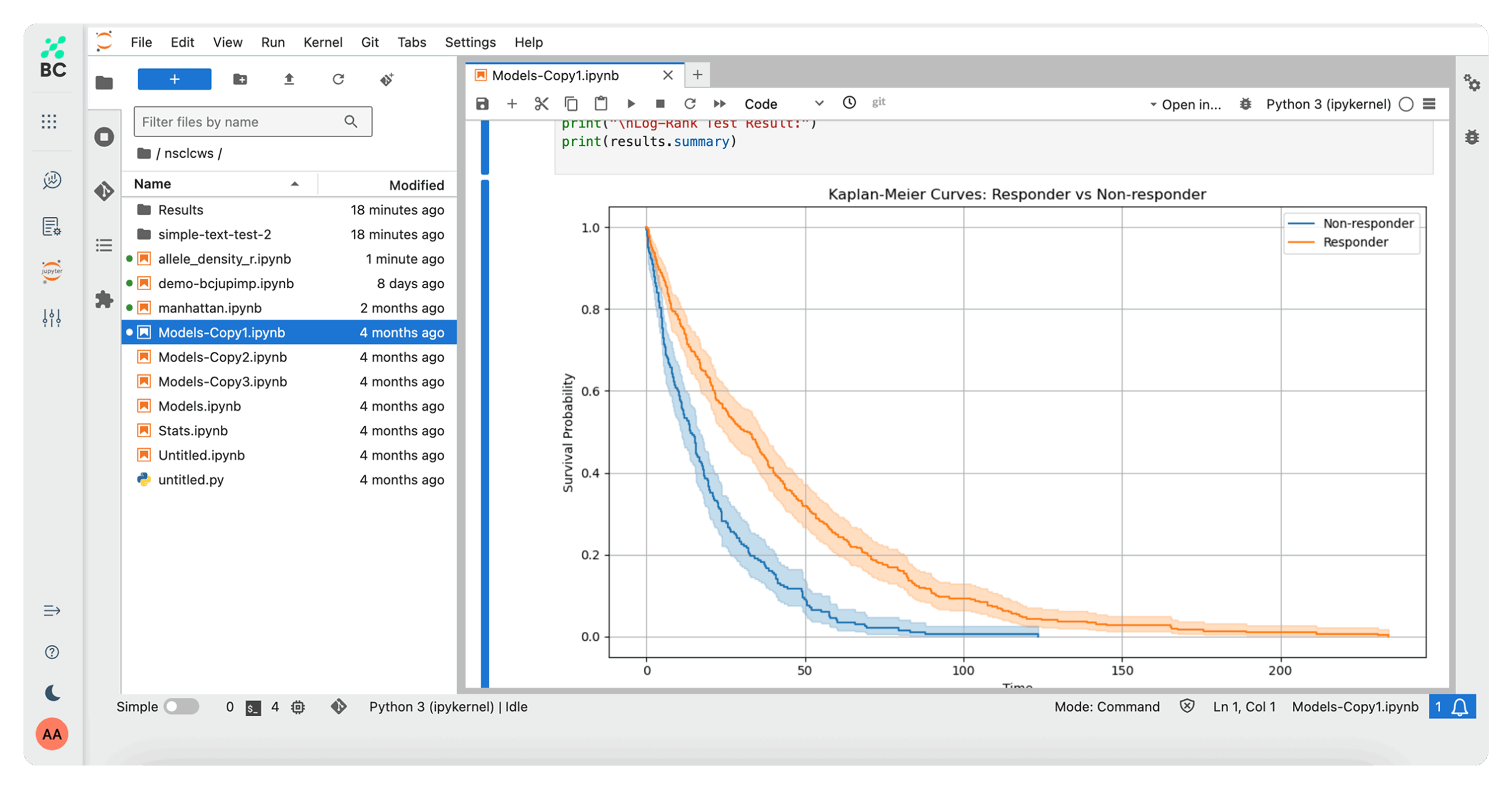
Work in an integrated analytical environment using reproducible, AI-powered workflows for exploring and processing large datasets to execute even the most comprehensive research studies.
Feel confident your information is secure with multi-tier, role-based access that lets you manage institutional and individual permissions on a project-specific basis.
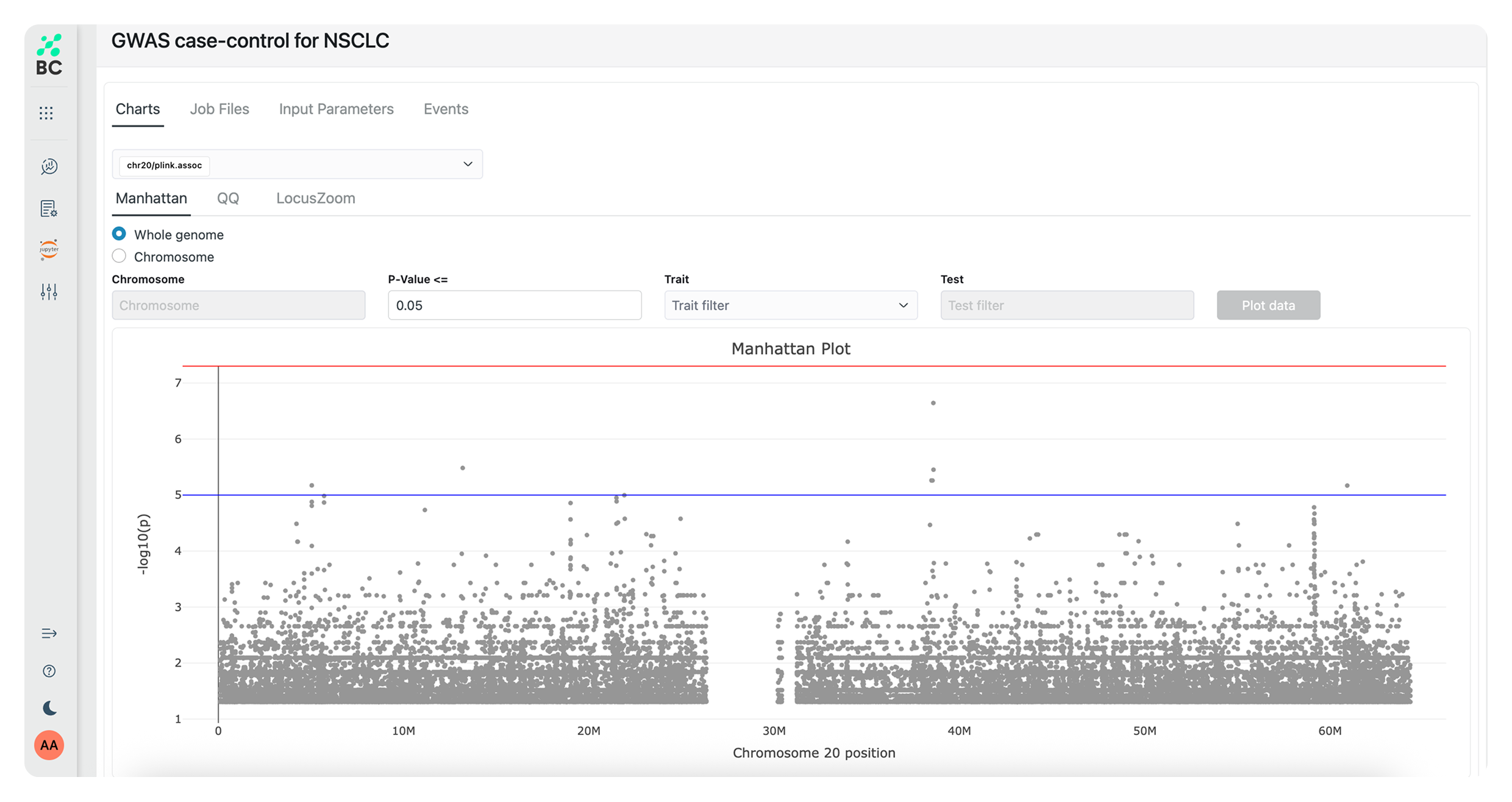
Effortlessly generate graphical reports and complex data visualizations, accelerating speed to insight as you identify trends and pinpoint key findings. Use predefined analysis pipelines – or create your own for specific study requirements.





BC Mosaic is powered by BC Unify, which acts as the integration engine behind our solutions — ingesting, enriching, and transforming diverse data into a harmonized structure that supports advanced analytics and healthcare research.
Spend less time organizing data and more time applying it. BC Mosaic serves up your data – and the advanced analytical tools you need – in a single, secure research environment.
Save time and automate complex tasks through our AI-powered data mastering and integration capabilities.
Tap into integrated multi-modal genomic and health data in a few clicks to generate deep patient and treatment insights.
Enable more proactive care and disease prevention. Uses statistical algorithms, machine learning, and AI to analyze historical data and forecast future health outcomes.
Use BC Mosaic to bridge the gap between data custodians and researchers – building trust, enabling secure collaboration, and providing essential tools for advancing innovation.
HEALTHCARE ORGANIZATIONS
Join our global data partner network and become part of a pioneering ecosystem that accelerates medical breakthroughs, enhances clinical decision-making, and drives personalized care across the most critical therapy areas.
LIFE SCIENCE COMPANIES
Access high-quality, diverse, harmonized real-world data to identify patient cohorts, assess treatment outcomes, and generate real-world evidence that supports clinical development, regulatory submissions, and market access.
BC Platforms meets the highest international standards for data protection, product quality, and ethical data governance. Our ISO-certified systems and compliance frameworks protect every stage of the data lifecycle, ensuring partners can innovate confidently and responsibly.
BC Platforms in the press: Signify Research reviews BC Catalyst, our AI-powered platform for life sciences and precision medicine.
Narasimha Kumar joins us as Global Head of Technology Consulting and India Country Head.
BC Platforms in the press: GeneOnline highlights our call to close Asia’s data gap and our role in building secure TREs.
Contact us to learn more about our solutions, see a live demo, or talk about how we can support your needs.